The Primary Role Of The Carbonic-acid-bicarbonate Buffer System Is To
The primary role of the carbonic-acid-bicarbonate buffer system is to. The primary role of the carbonic-acid-bicarbonate buffer system is to - 13451581 fakeemial8014 fakeemial8014 10042019 Chemistry High School answered The primary role of the carbonic-acid-bicarbonate buffer system is to 1 See answer fakeemial8014 is waiting for your help. What is the primary role of the carbonic acid-bicarbonate buffer system. If youre seeing this message it means were having trouble loading external resources on our website.
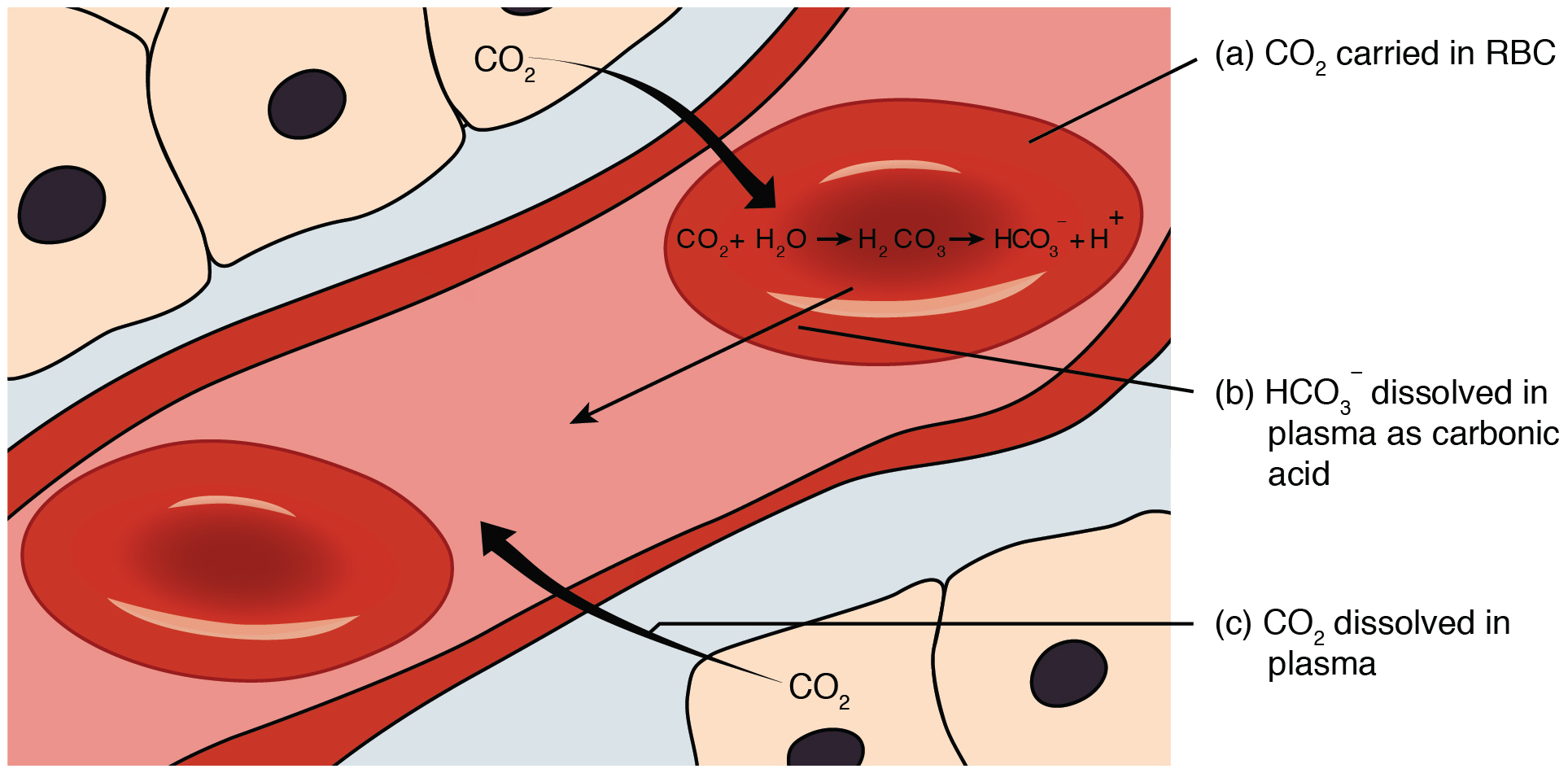
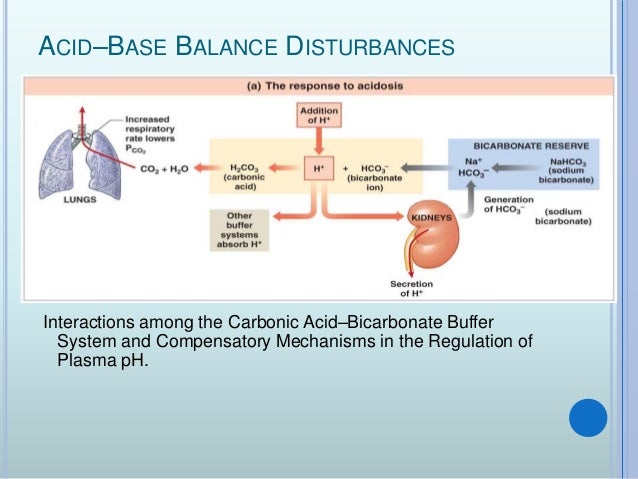
Buffers are also effective at higher concentration. In biochemistry the name carbonic acid is often applied to aqueous solutions of carbon dioxide which play an important role in the bicarbonate buffer system used to. The respiratory and renal systems also play major roles in acid-base homeostasis by removing CO2 and hydrogen ions respectively from the body.
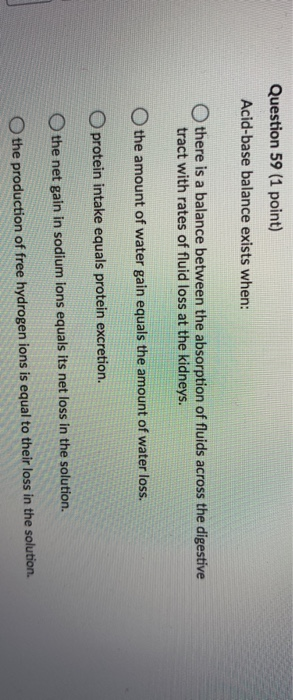
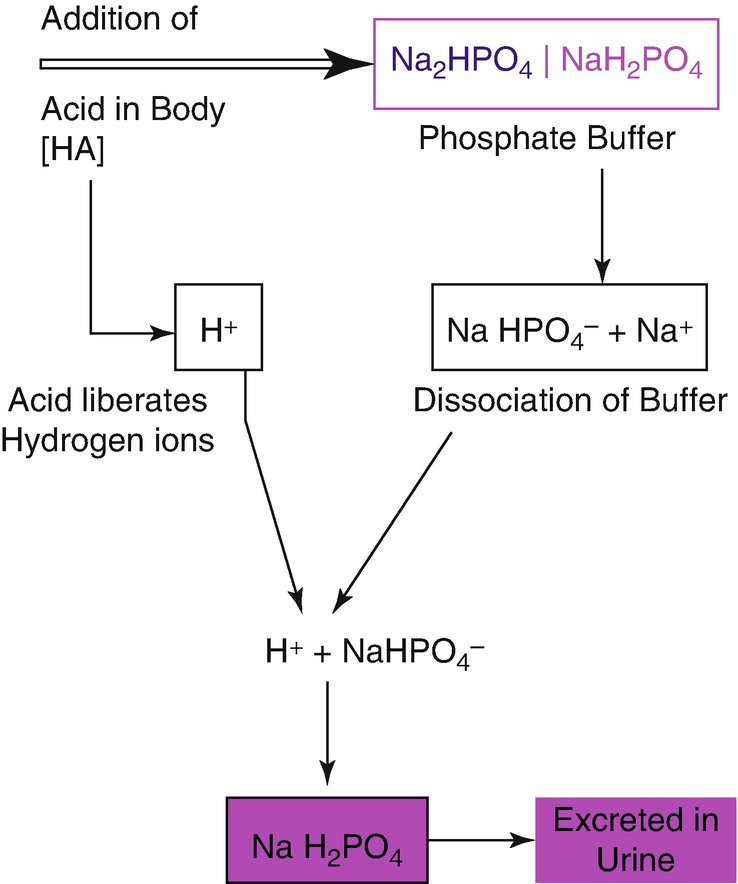
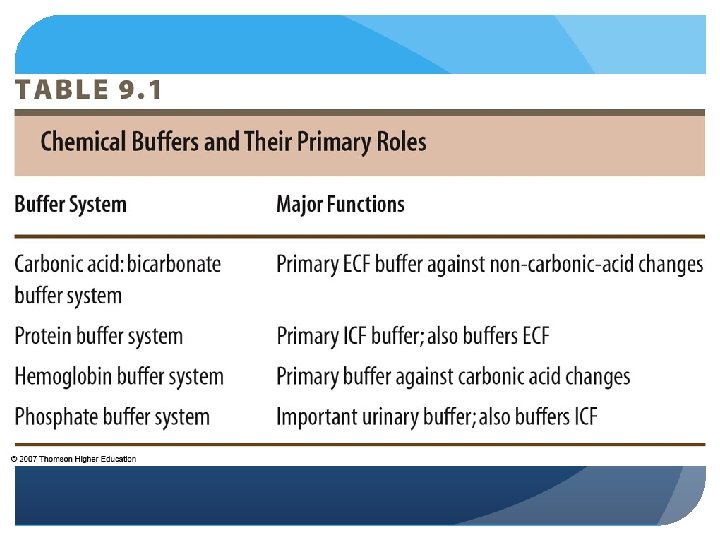
Several substances serve as buffers in the body including cell and plasma proteins hemoglobin phosphates bicarbonate ions and carbonic acid. If acid and base components of buffer are equal the pH is equal of pK. The primary role of the carbonic acidbicarbonate buffer system is to _____.
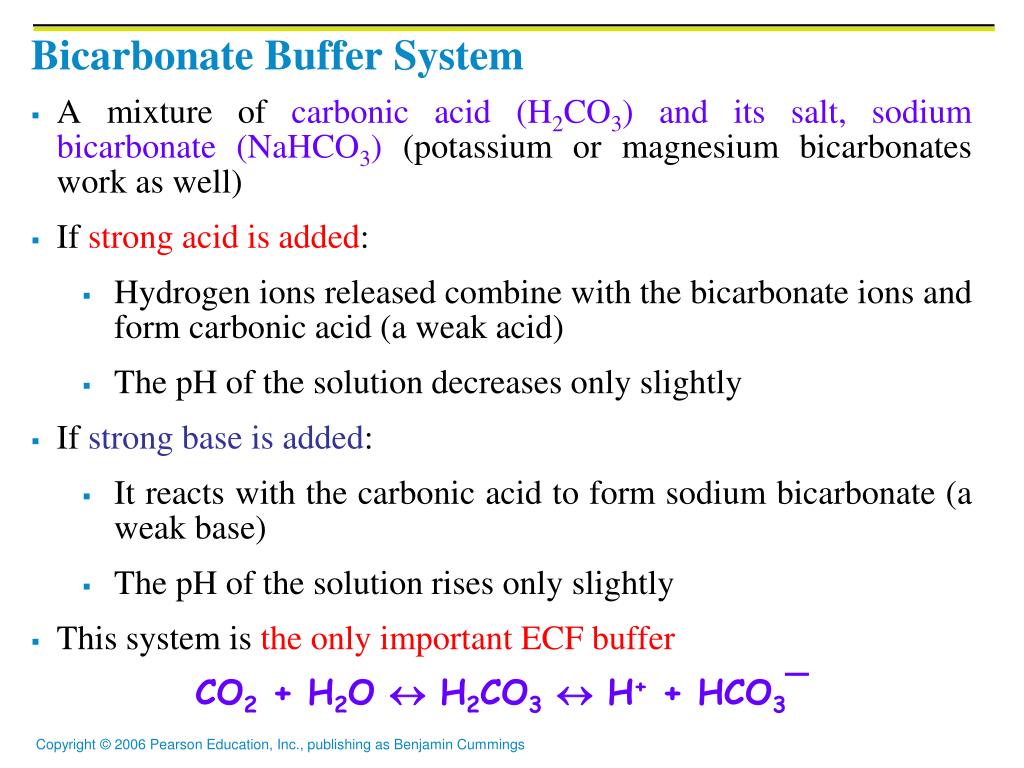
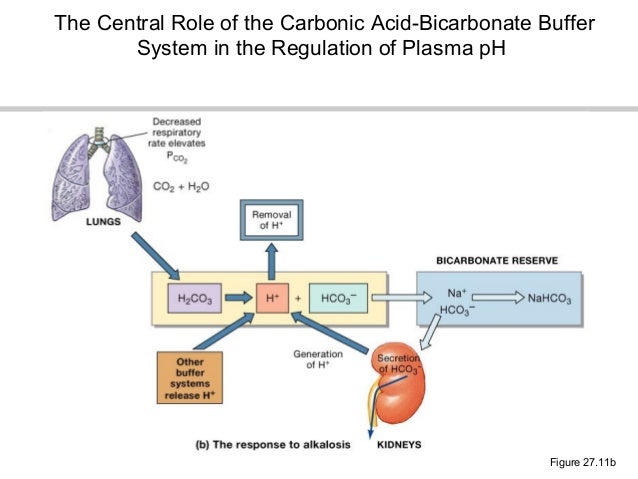
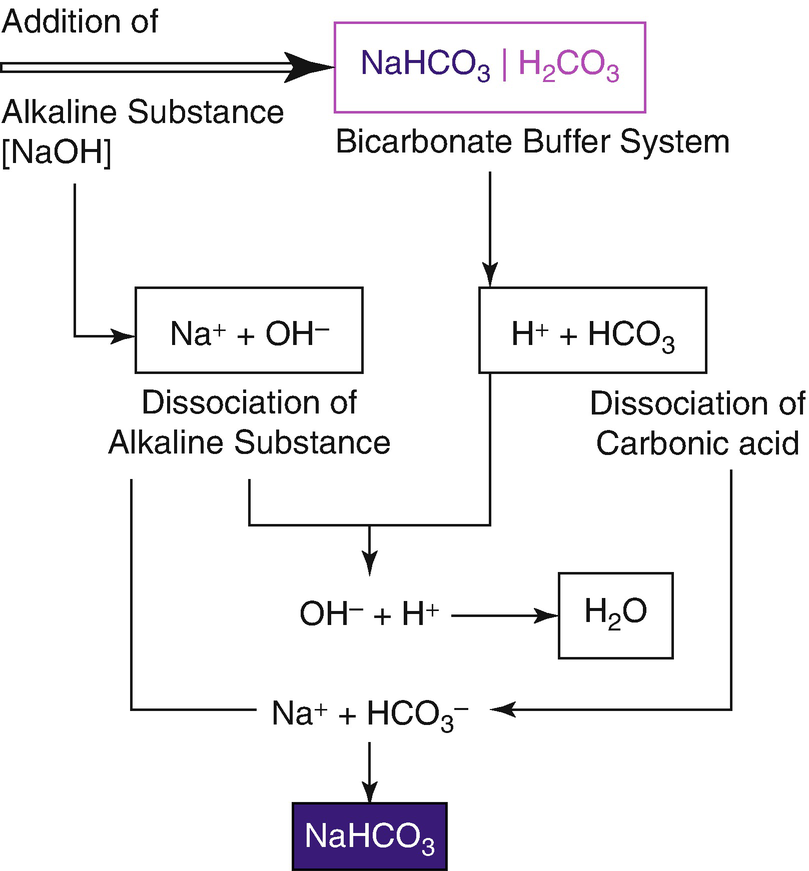
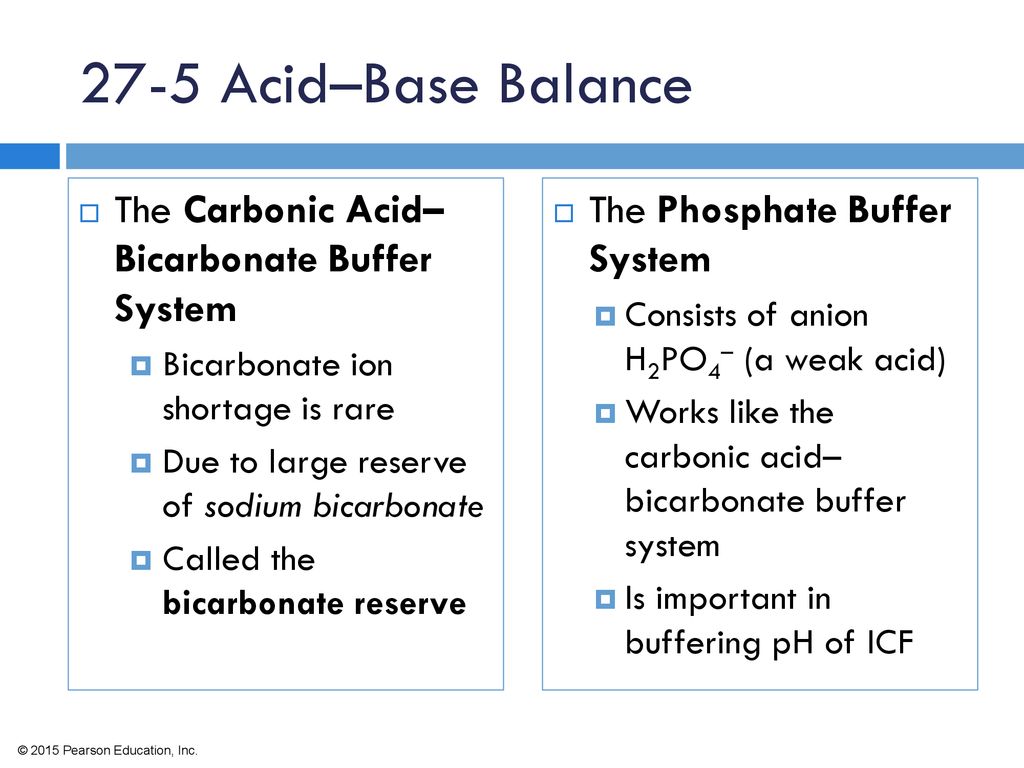
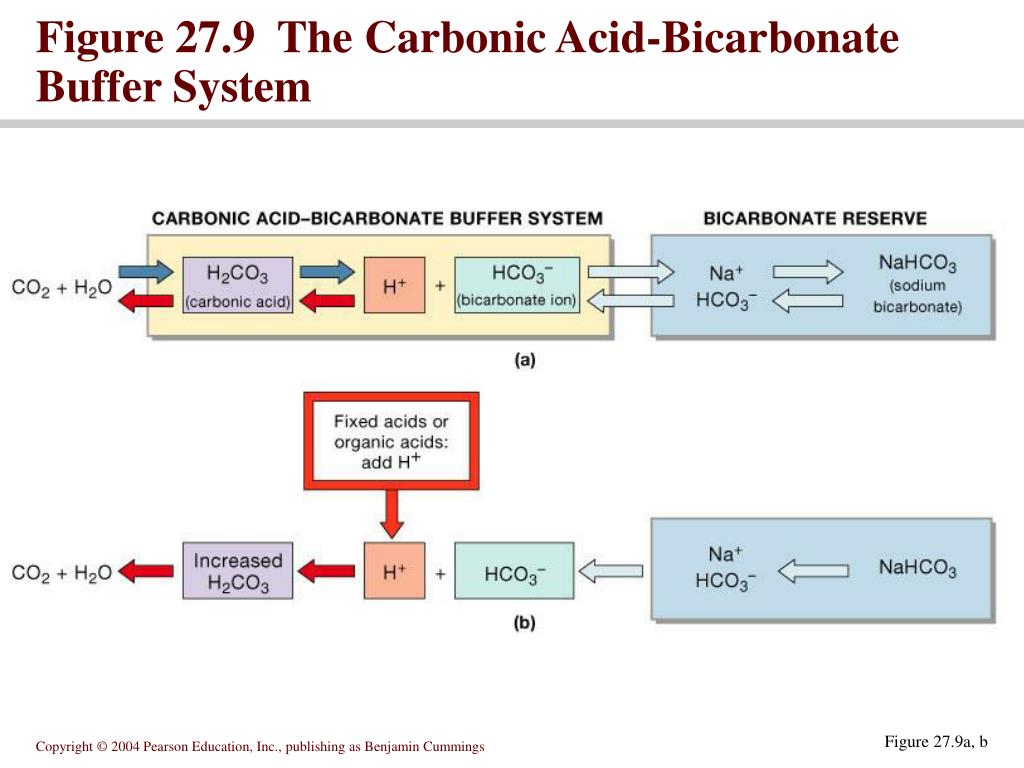
The bicarbonate buffer system is an acid-base homeostatic mechanism involving the balance of carbonic acid H 2 CO 3 bicarbonate ion HCO. The carbonatecarbonic acid buffer the phosphate buffer and the buffering of plasma proteins. The role of the bicarbonate buffer system in regulating blood pH practice Khan Academy.
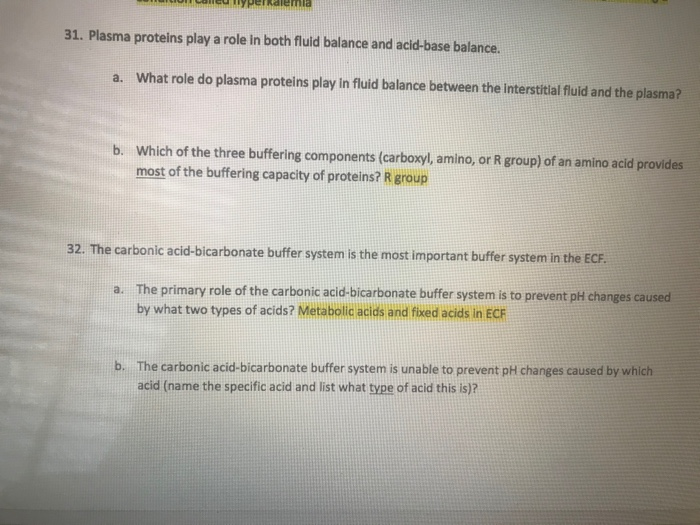
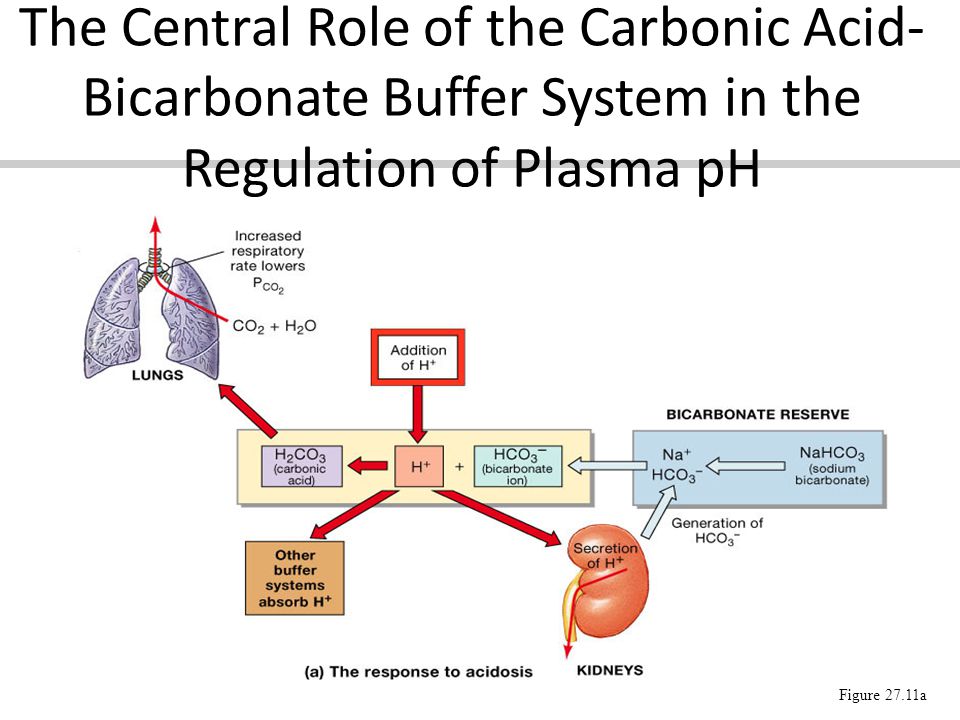
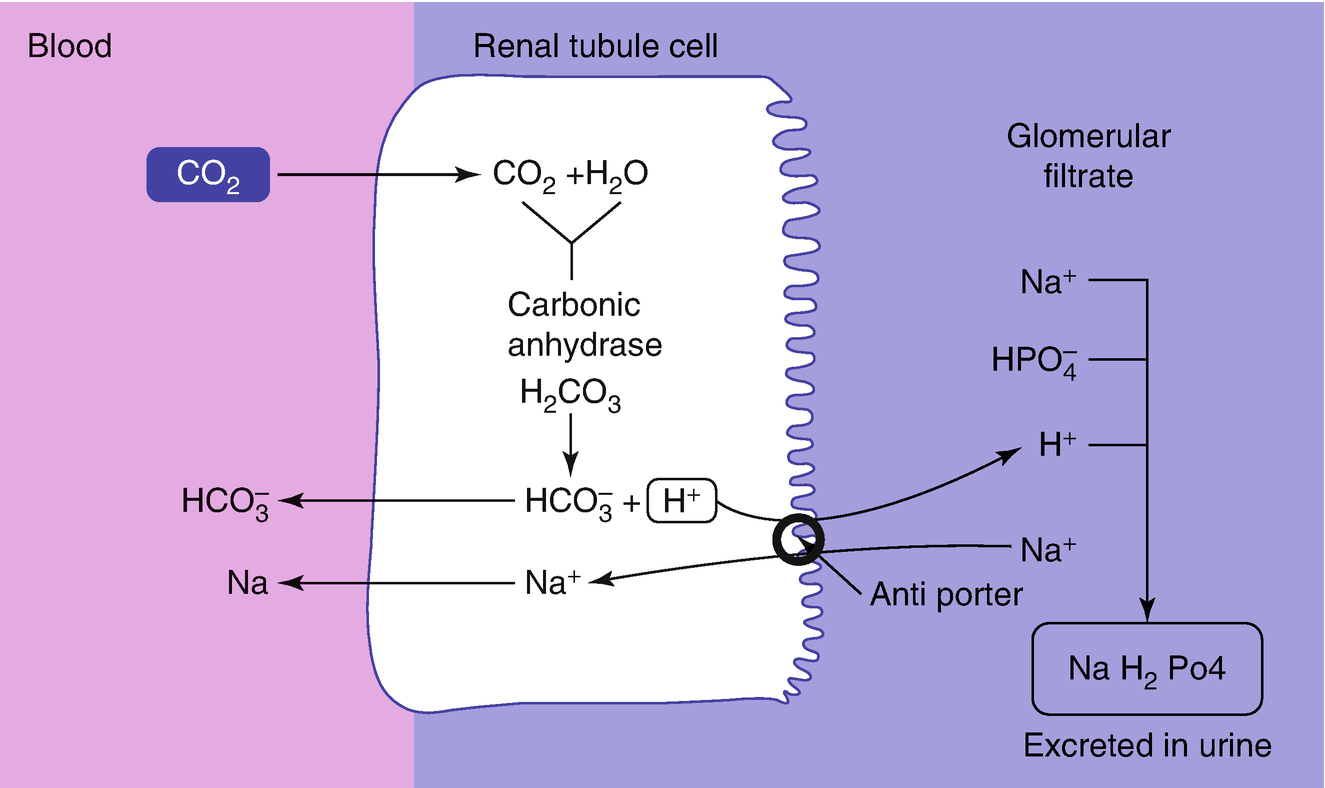
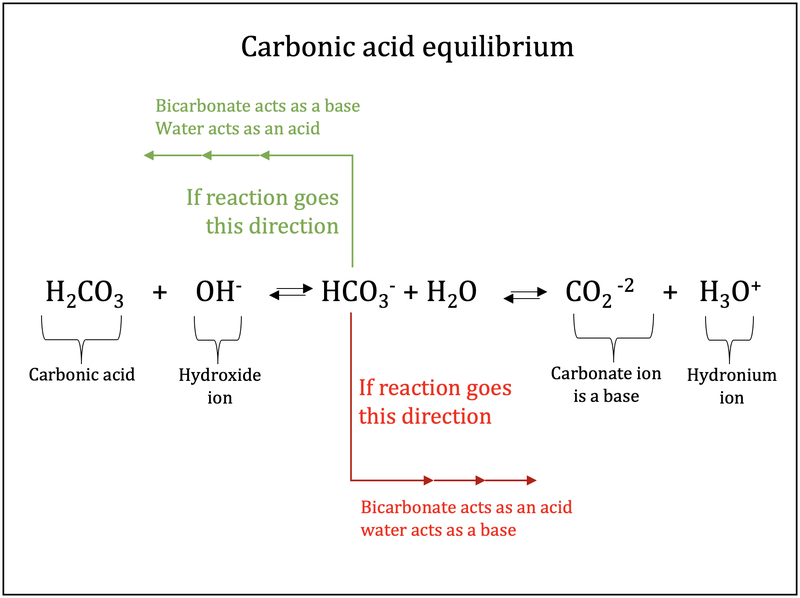
The bicarbonate buffer is the primary buffering system of the IF surrounding the cells in tissues throughout the body. The primary role of the carbonic-acid-bicarbonate buffer system is to ANSWER. The important thing to realize here is that carbonic acid H 2CO3 is actually formed when carbon dioxide CO2 is dissolved in water.
The primary role of the carbonic acid-bicarbonate buffer system is asked Nov 26 2018 in Anatomy Physiology by HoshGosh A to buffer carbonic acid formed by carbon dioxide. While the third buffer is the most plentiful the first is usually considered the most important since it is coupled to the respiratory system. Base is within the range of 10.
The respiratory and renal systems also play major roles in acid-base homeostasis by removing CO 2 and hydrogen ions respectively from the body. To help buffer blood pH.
The primary role of the carbonic acidbicarbonate buffer system is to _____.
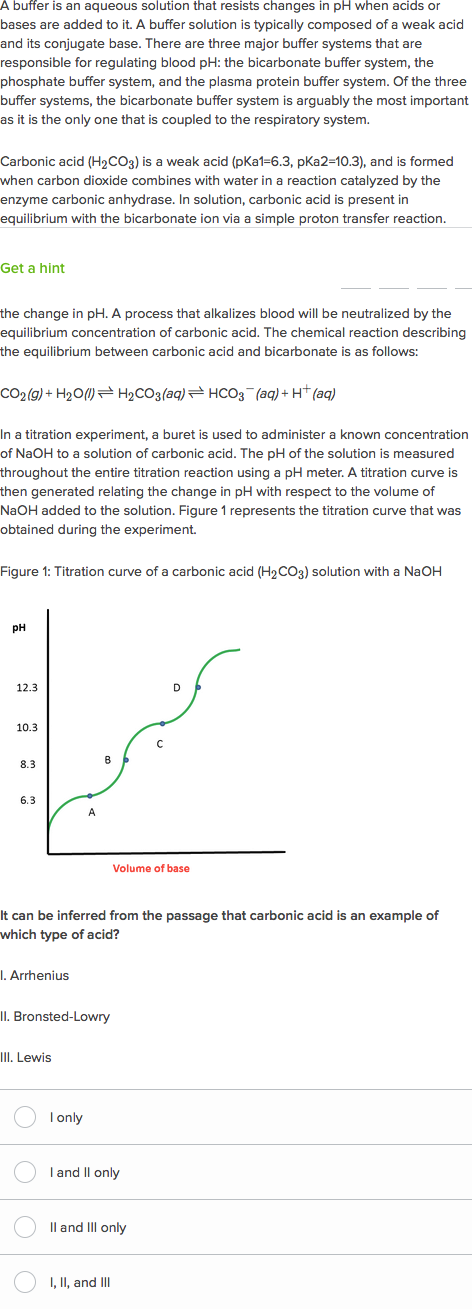
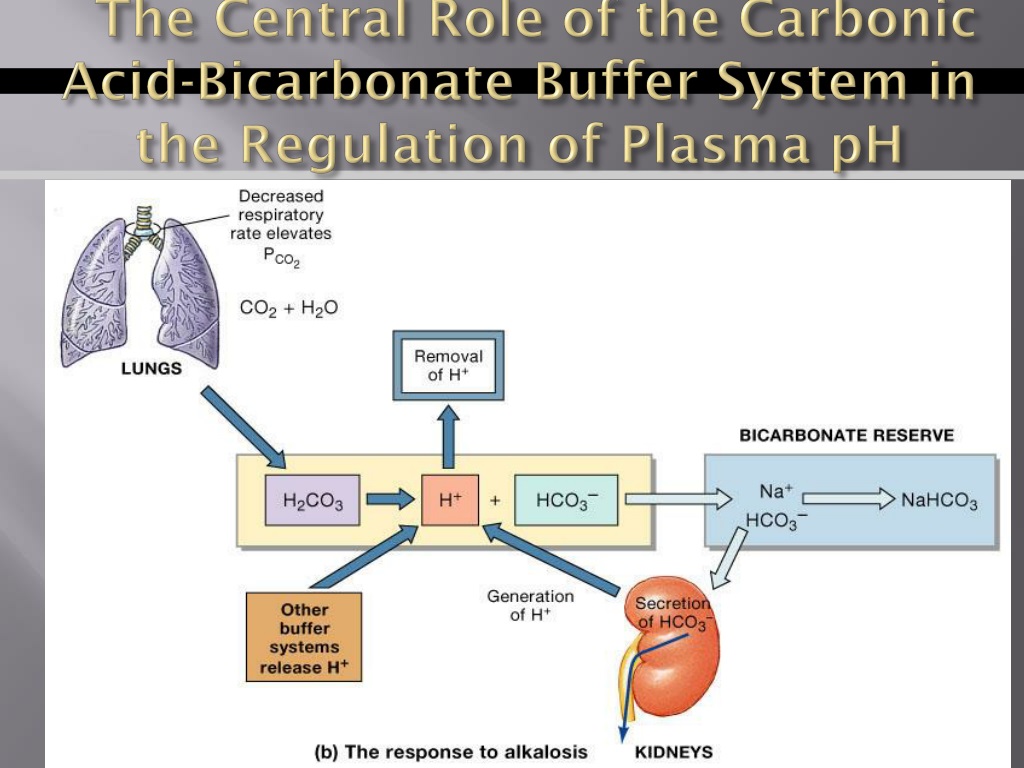
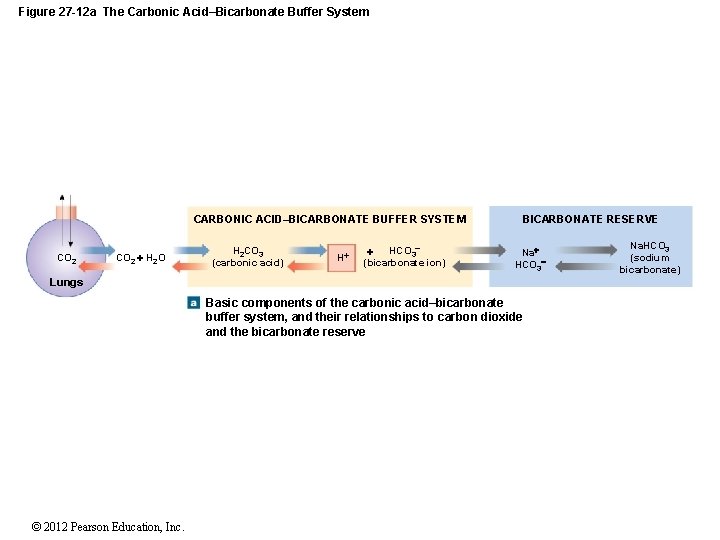
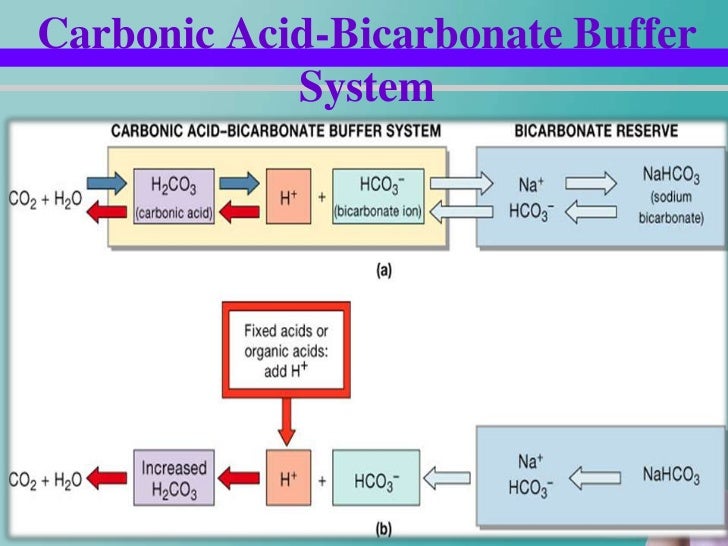
Buffer carbonic acid formed by carbon dioxide. If acid and base components of buffer are equal the pH is equal of pK. The carbonic acid - bicarbonate buffer system consists of carbonic acid a weak acid and the bicarbonate anion its conjugate base. Together with other buffering systems it is responsible for keeping blood pH at physiological values. The primary role of the carbonic acid-bicarbonate buffer system is to b buffer carbonic acid formed by carbon dioxide. The primary role of the carbonic-acid-bicarbonate buffer system is to ANSWER. It alsoprotects against the. The respiratory and renal systems also play major roles in acid-base homeostasis by removing CO2 and hydrogen ions respectively from the body. The carbonatecarbonic acid buffer the phosphate buffer and the buffering of plasma proteins.
The primary role of the carbonic acid-bicarbonate buffer system is asked Nov 26 2018 in Anatomy Physiology by HoshGosh A to buffer carbonic acid formed by carbon dioxide. The primary role of the carbonic-acid-bicarbonate buffer system is to ANSWER. The role of the carbonic acid-bicarbonate buffer system is to help regulate and maintain the homeostatic pH balance within the blood. If youre seeing this message it means were having trouble loading external resources on our website. The important thing to realize here is that carbonic acid H 2CO3 is actually formed when carbon dioxide CO2 is dissolved in water. To help buffer blood pH. The primary role of the carbonic acidbicarbonate buffer system is to _____.


























Post a Comment for "The Primary Role Of The Carbonic-acid-bicarbonate Buffer System Is To"